Gene editing: can the law keep up?
Tom Bangay
The use of revolutionary gene editing techniques to enhance our knowledge of infertility, pregnancy and disease is an expanding market, attracting investor interest and hype among the scientific community. But, while so far tightly controlled, its growing application raises various legal and ethical questions.
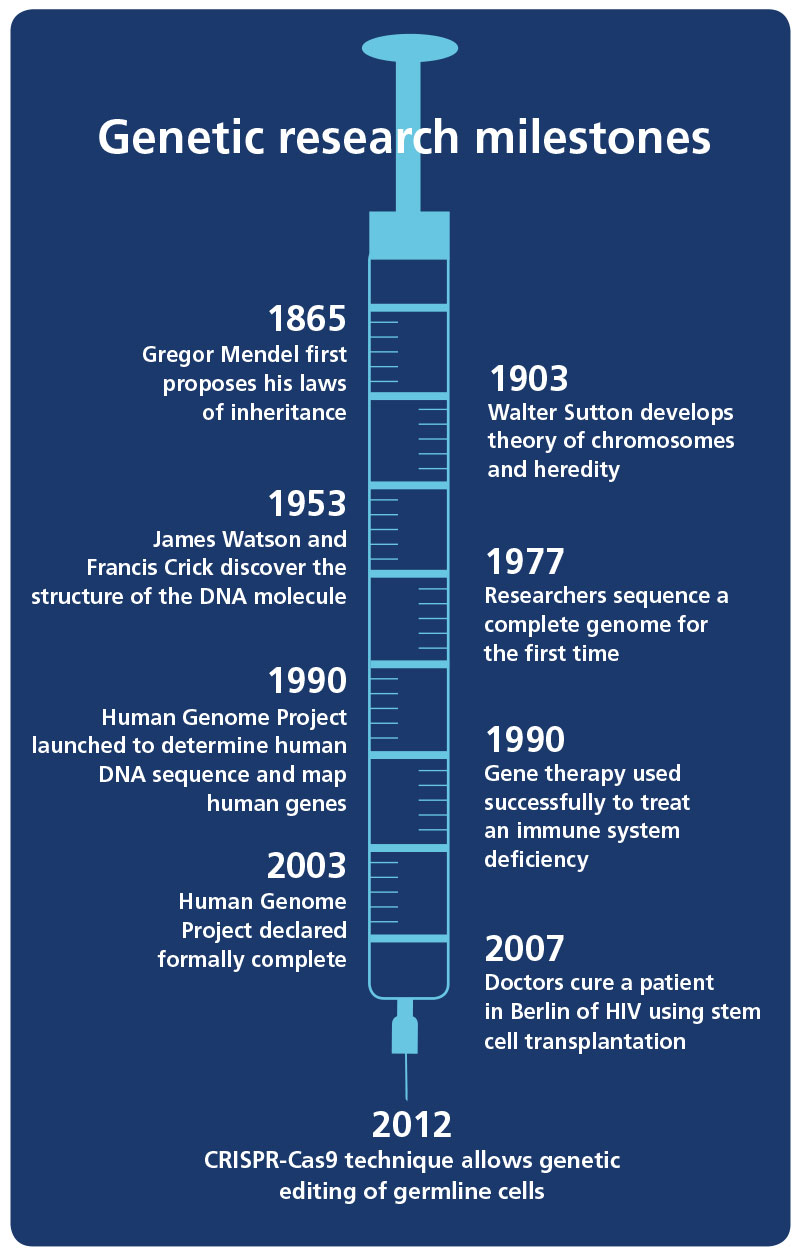
In 1994, Francis Collins, the American geneticist who led the Human Genome Project, likened trying to find the gene responsible for a disease to ‘trying to find a burned-out light bulb in a house located somewhere between the East and West coasts without knowing the state, much less the town or street the house is on’.
Genomics has come a long way since then. The mapping of the human genome was completed in 2003. Four years later, doctors in Germany cured a patient of HIV using stem cell transplantation. And now, the latest revolutionary scientific technique – low-cost, targeted gene editing – has the potential to drive our understanding of and ability to manipulate genetics to previously unthinkable heights.
In January 2016, a group led by Dr Kathy Niakan at the Francis Crick Institute in London was granted consent by the United Kingdom’s Human Fertilisation and Embryology Authority (HFEA) to undertake germline gene editing using human embryos. If successful, the project could profoundly improve our knowledge of infertility, early development, miscarriage and the treatment of serious diseases and medical conditions.
Better that it’s done openly, with proper academic and ethical discussion, than somewhere less regulated
Patricia Barclay
Founder of Bonaccord and Chair of the IBA Healthcare and Life Sciences Law Committee
‘I believe our research could ultimately lead to improvements in infertility treatment and provide a deeper understanding of the earliest stage of human life,’ said Dr Niakan. ‘I would like to understand the role of genes that we think are likely to be very important for healthy development and for stem cells. If we could study these genes, they could be used as a biomarker to identify those embryos that would go on to develop successfully into a healthy baby.’
Globally, the estimated rate of miscarriage is between 15 and 20 per cent among women who know they’re pregnant. ‘The knowledge we acquire will be very important for understanding how a healthy human embryo develops, and this will inform our understanding of the causes of miscarriage. It may also improve embryo development after in vitro fertilisation (IVF), and might provide better clinical treatments for infertility,’ added Niakan.
Previous applications of gene editing techniques have focused on somatic (non-reproductive) cells, meaning that any effects of gene editing would be confined to the individual subject in question. However, as Patricia Barclay, founder of Bonaccord and Chair of the IBA Healthcare and Life Sciences Law Committee, explains: ‘What’s new here is that they’ve been given permission to edit germline cells, which could adjust a defective gene for future generations.’ What if the potential for a devastating illness could be identified and fixed before a child were even to be conceived?
Revolutionary technique
The research makes use of CRISPR-Cas9, a highly efficient, relatively low-cost gene editing technique, which came to prominence in 2012 and by the end of 2014 was cited in some 600 research papers. ‘It has revolutionised the way gene editing is carried out, and numerous gene editing companies using this technology have exploded onto the scene,’ says Dr Sahar Shepperd, Associate in Intellectual Property and Commercial Transactions at Bristows.
The scope for swift progress is enormous and has generated much excitement in the global academic community. Commercial partners have also been keen to make early investments in a technology with vast growth potential: a MarketsandMarkets report projected the value of the global genome editing market to reach $3.5bn by 2019. Time magazine named Dr Niakan as one of the world’s 100 most influential people for 2016 in recognition of her groundbreaking experiments.
However, controversy inevitably accompanies any new development where genes and embryos are concerned. Dr David King, Director of Human Genetics Alert, called this ‘the first step in a well mapped-out process leading to genetically-modified babies and a future of consumer eugenics’.
US President Obama’s science adviser, John Holdren, called human germline editing ‘a line that should not be crossed at this time’.
What is CRISPR-Cas9?
Clustered regularly interspaced short palindromic repeats – abbreviated to CRISPR – are the segments of DNA that lie at the heart of the new technique revolutionising genomics. The simplified Cas9 technique was pioneered by Jennifer Doudna from the University of California, Berkeley and Emmanuelle Charpentier of the Helmholtz Centre for Infection Research in Germany, and first demonstrated in 2012.
The use of the Cas9 protein enables editors to find a specific target within DNA. In 2013, they used it to cut out a section of DNA in human cells, replacing it with another piece. As well as cutting up DNA, a team at Stanford University have found ways to use Cas9 to control genes, rather than altering and replacing them. A key element of CRISPR and why its implications raise so many questions, is that the edits it allows in genes can be passed to ancestors.
Doudna and Charpentier each received $3m for winning the Breakthrough Prize in Life Sciences in 2015, an award sponsored by a group of tech billionaires. CRISPR-Cas9 has been the subject of much hype in the industry, but its real-world applications are well into development. Novartis, AstraZeneca, Vertex and Regeneron are among the firms making big commercial bets on the technique. Startup Editas, founded by CRISPR-Cas9 patent holder Feng Zhang, has raised more than $160m in funding.
The International Summit on Human Gene Editing, held in Washington, DC in December 2015, allowed scientists and policymakers to air such concerns publicly. Daniel Kevles, formerly of the California Institute of Technology and Yale University, set out perhaps the crucial question: ‘What seemed like a moral or technical issue in the past is, in this society, very likely to become a consumer question of who can afford it. Will parents want to use germline modification to enhance a child’s genetic endowment?’
Behind the somewhat alarmist public discourse, the reality is that the consent for the Francis Crick Institute’s project is extremely narrow and tightly controlled. James Lawford Davies, a partner at Hempsons, advised the Institute on the application for the licence to carry out gene editing. ‘The regulatory responsibility for clinics and research centres is quite significant, and the law and regulations are complex and voluminous,’ he says. In this case, Dr Niakan’s team are working with donated embryos left over from IVF treatment, which cannot be used after 14 days and are prohibited from ever being implanted in a woman.
These mandatory requirements are enshrined in the UK’s Human Fertilisation and Embryology Act 1990 which, when combined with the HFEA Code of Practice, sets out a framework for new technologies that is both flexible and unambiguous.
Lawford Davies explains: ‘The UK doesn’t often regulate specific technologies or procedures. Regulations tend to be drafted far more broadly and relate to the purpose of the research and treatment and the material used. This allows a broad degree of flexibility to the regulator in deciding whether or not to grant a licence.
‘So, for example, it’s very clear from the UK law that the Crick Institute’s work on gene editing is permitted, but you’ll never find the phrase “gene editing” in UK law. Conversely, the use of gene edited embryos is very obviously prohibited, despite not being specifically mentioned in UK law.’
This is underlined by the fact that the HFEA has 18 currently licensed research projects involving human embryos, including the Francis Crick Institute’s – indicating that this type of research isn’t as startling and unprecedented as headlines might suggest.
Regulated but permissive
Barclay agrees that the UK regime is ‘regulated but permissive’ and, as a fairly secular state, the UK authorities tend to make decisions in a scientific, unemotional way.
She adds that the rapid advancement of CRISPR-Cas9 means that ‘if we don’t do it, another country will – better that it’s done openly, with proper academic and ethical discussion, than done in a back shop somewhere less regulated’. Dr Niakan said that ‘the HFEA has been instrumental in establishing a culture of proper discourse and regulatory oversight.
Each jurisdiction’s reaction to gene editing will depend on its legal system, as well as the political and religious considerations that might be at play in some countries. A recent poll conducted by STAT and Harvard TH Chan School of Public Health found that 65 per cent of the public in the United States believes that gene editing to reduce the risk of an unborn child developing certain serious diseases should be illegal. However, against this backdrop, in June 2016, a team of researchers from the University of Pennsylvania received approval from a federal committee to launch a trial to study the safety of CRISPR-Cas9 on human patients. US Food and Drug Administration approval is considered a formality, and trials could begin soon.
The commercialisation and development of gene editing in the US has also run up against a predictable obstacle: litigation. On 15 March 2013, CRISPR-Cas9 pioneer Professor Jennifer Doudna, of the University of California, Berkeley, filed for a patent on the invention of a way to use the technique to edit the genomes of cells in higher organisms. Seven months later, Feng Zhang of the Broad Institute and the Massachusetts Institute of Technology filed for his patent. However, on 16 March – a day after Doudna filed – the US changed its patent filing system to harmonise with other countries, meaning that the moment of invention replaces the moment of filing as the key date.
The question of who invented the technique first is set to be decided by the US Patent and Trademark Office, with oral arguments scheduled for December 2016. The rewards on offer for victory – both in terms of licensing revenue and prestige – are huge, in what could be one of the biggest biotech cases for years.
Elsewhere, a team of Chinese scientists claimed in April 2015 to have modified a gene linked to blood disease in human embryos. In April 2016, a second team, led by stem cell scientist Yong Fan, published a paper claiming to have used CRISPR-Cas9 technology to modify a specific immune cell with the aim of making a human embryo immune to HIV infection. Fan’s team was quick to reassure a public who may feel that science is moving too fast. ‘We believe that any attempt to generate genetically-modified humans through the modification of early embryos needs to be strictly prohibited until we can resolve both ethical and scientific issues,’ he wrote.
That ethical debate will be vital to the future viability of gene editing, particularly in an international regulatory environment struggling to keep up. Juliet Tizzard, the HFEA’s Director of Strategy and Corporate Affairs, stresses that as far as the UK is concerned, ‘what other countries are doing is irrelevant to our licensing decisions’. However, she does acknowledge that ‘technology does sometimes move faster than the law, though in the example of gene editing, the law already permits this in research’.
Balancing scientific progress with public approval is always a key aim: ‘We try to keep our policies balanced between the interests of patients and researchers/research – and we take time to talk to people working in laboratories, in clinics and to patients,’ explains Tizzard. ‘These kinds of relationships create more public trust than a government department alone might be able to achieve.’ Lawford Davies agrees: ‘Maintaining an open dialogue between researchers, public and the media is very important in maintaining confidence.’
Not every jurisdiction will approach gene editing with the same scrutiny, and the risk of its techniques being used to pursue results never foreseen by its creators is real and troubling. ‘There’s no doubt that CRISPR will eventually be used in a way that we’d never imagine being permitted here,’ cautions Lawford Davies. ‘That’s been the experience with stem cell research and it can dent the confidence of the public and the press.
‘In the Caribbean, for example, you can have foetal stem cells injected into your forehead for cosmetic purposes in a way that would be criminal in the UK. There’s a real difficulty in how the global research community addresses the possibility of therapeutic tourism. The risk is that it’s only a matter of time before there’s a jurisdiction where this is used irresponsibly, which will be damaging to its responsible use in other jurisdictions.’
There’s a real difficulty in how the global research community addresses therapeutic tourism
James Lawford Davies
Partner at Hempsons
Meanwhile, the legal response to advances in science continues apace, with different countries having different levels of flexibility to accommodate new techniques. Chris Holder, a partner at Bristows and Vice Chair of the IBA Technology Law Committee, offers a reminder of one of the advantages of our common law jurisdiction: ‘When you’ve got the concept of negligence, you don’t have to write a specific law, because companies owe the wider public a duty of care. You don’t have to create a regulatory framework for every new technology. Civil law colleagues on the continent are more likely to consider regulation when dealing with new technology, rather than relying on existing legal principles supplemented by legislation.’
Dr Niakan has received approval from the Cambridge Central Research Ethics Committee and her work is now in development, pending a sufficient supply of embryos. Obtaining unused embryos from IVF treatments is not a simple process, with patients sometimes feeling uncertainty or anxiety about donating, but the Francis Crick Institute offers counselling to donors and its patient consent forms are rigorously detailed.
CRISPR-Cas9 and gene editing generally will continue to push forward, both here and abroad: ‘The next decade or so will demonstrate how the power of this technology is harnessed to eradicate genetic abnormalities on a permanent basis,’ says Shepperd. ‘There may be a need for regulations on a cross-border level, but this will take time and a significant amount of debate, reasoning and analysis.’
Tom Bangay is a freelance journalist and can be contacted on thomas.bangay@gmail.com